Sec4p·Sec2p 複合体
Vesicular transport during exocytosis is regulated by Rab GTPase (Sec4p in yeast), which is activated by a guanine nucleotide exchange factor (GEF) called Sec2p.
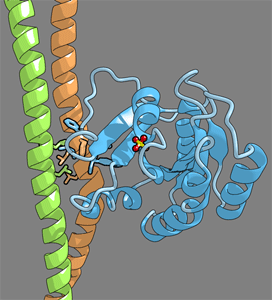 |
We solved the crystal structure of the Sec2p GEF domain in a complex with the nucleotide-free Sec4p at 2.7 Å resolution. Unlike the recently reported 3.3 Å structure of the Sec4p·Sec2p complex, our structure contains a phosphate ion bound to the P-loop, which may represent an intermediate state of the nucleotide exchange reaction. |
Upon complex formation, the Sec2p helices approach each other, flipping the side chain of Phe-109 toward Leu-104 and Leu-108 of Sec2p. These three residues provide a hydrophobic platform to attract the side chains of Phe-49, Ile-53, and Ile-55 in the switch I region as well as Phe-57 and Trp-74 in the interswitch region of Sec4p. Consequently, the switch I and II regions are largely deformed, to create a flat hydrophobic interface that snugly fits the surface of the Sec2p coiled coil. These drastic conformational changes disrupt the interactions between switch I and the bound guanine nucleotide, which facilitates the GDP release.
![[XXX]](/image/tw.png)